Next Generation TCR
therapeutics for solid
cancer
Towards effective options for patients suffering from difficult to treat tumors
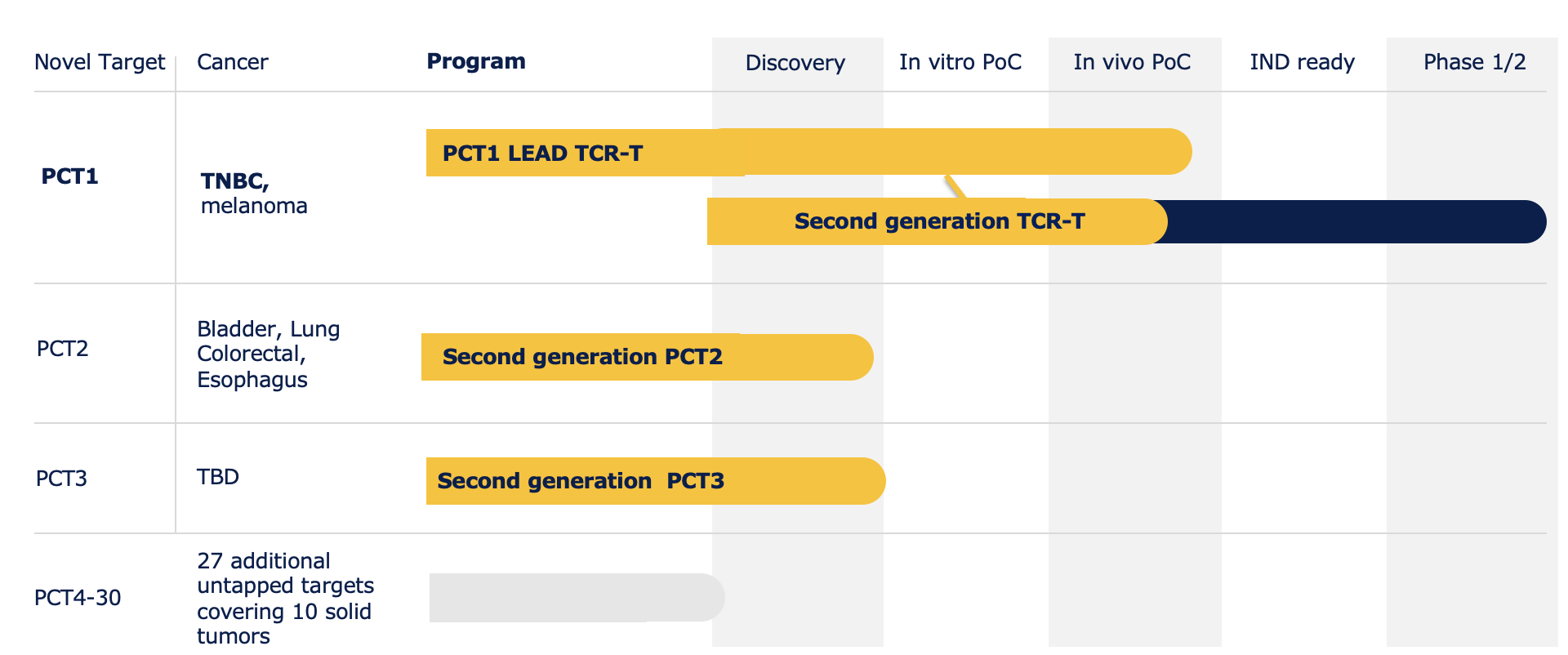
Pan Cancer T is developing a broad pipeline of TCR therapeutics directed against 10 major solid tumors. The lead clinical program, PCT-1, has demonstrated safety and efficacy in several tumor models, focusing on triple-negative breast cancer and melanoma. It serves as a spearhead to build capabilities, from research to clinic and establish a footprint.
Other oncology indications in preclinical programs are bladder, ovarian, lung, esophagus, colon, prostate, and glioma.
Other oncology indications in preclinical programs are bladder, ovarian, lung, esophagus, colon, prostate, and glioma.